Targeted Therapy for Rare Neurological Diseases
- Home
- Neurological Disease
The Power of LNP mRNA Precision Medicine
Leveraging Proven Technology to Transform Gene Therapy for Rare Diseases & CNS Disorders
What is LNP & how does it work:
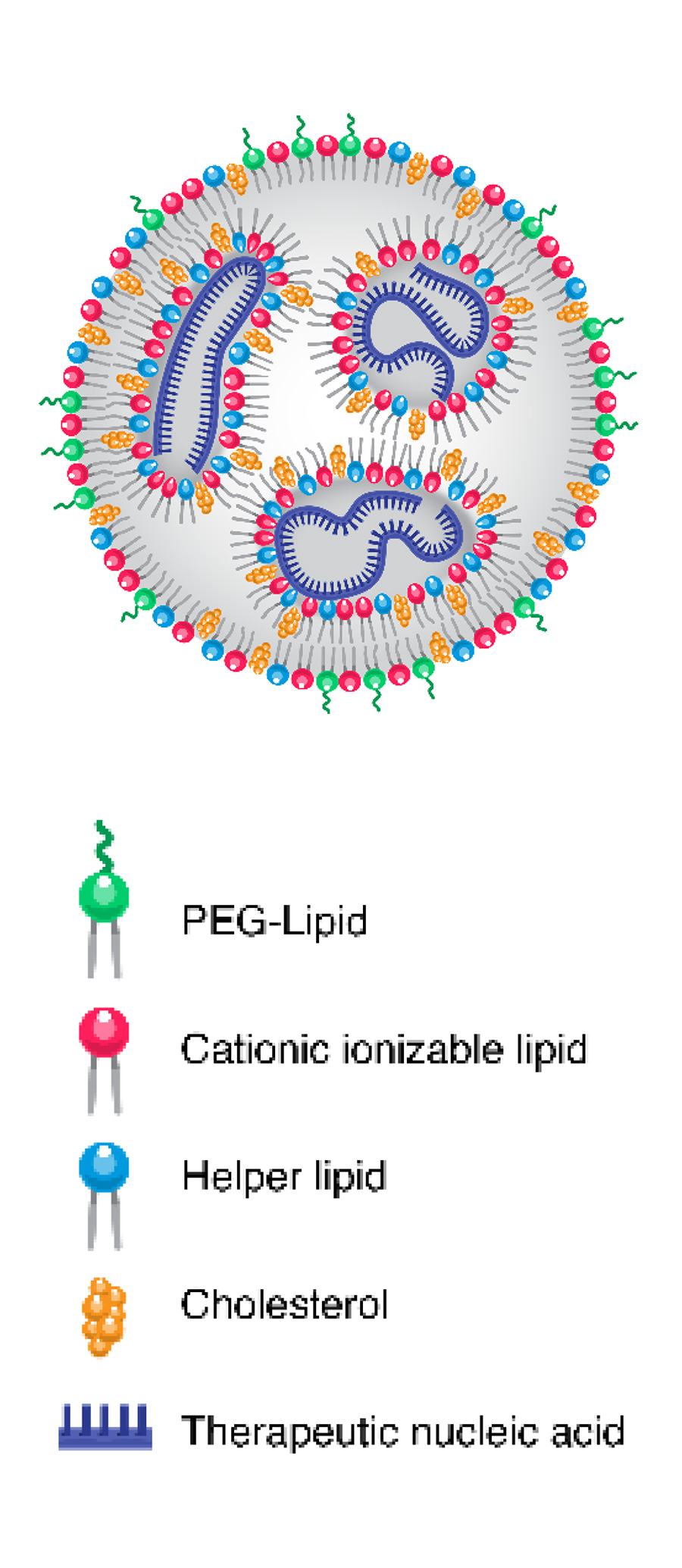
Lipid nanoparticles (LNPs) are delivery vehicles composed of biocompatible lipids (fats) that encapsulate therapeutic agents. They offer important therapy advantages:
- Biocompatibility: Their lipid composition closely resembles biological membranes, minimizing immune system reactions and toxicity.
- Versatility: They can be customized to encapsulate various types of therapeutic agents for diverse applications.
- Targeted Delivery: They can be functionalized with targeting ligands to specifically bind to receptors on cells in the central nervous system (CNS), enhancing drug delivery and reducing side effects.
- Controlled Release: They can be designed to release their cargo gradually over time, providing sustained therapeutic effects.
What is mRNA & how does it work:
- mRNA (messenger ribonucleic acid) is a molecule that carries instructions from DNA in the nucleus to ribosomes in the cytoplasm, telling them which proteins to build.
- The mRNA is encased in lipid particles that protect it and help it enter cells. Once inside the targeted cell, the mRNA instructs the cell's machinery to make copies of specific proteins.
- mRNA was first used in COVID-19 vaccines with great success.
Unleashing the power of mRNAs
Delivering Functional POLR3A mRNA Directly to Cells to Product Healthy Proteins
A Targeted LNP mRNA Therapy for 4H Leukodystrophy
Caused by POLR3A mutations leading to insufficient RNA polymerase III enzyme impacting protein production and causing severe neurological degeneration, 4H Leukodystrophy is primarily a pediatric disease with no cure. We aim to deliver mRNA that codes for therapeutic functional proteins, potentially restoring function and replacing missing proteins. Our proprietary LNP formulations enhance stability and intracellular mRNA release, maximizing therapeutic efficacy.
Restoring Myelin Function
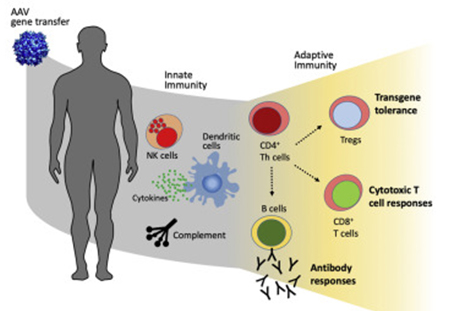
Combating Adrenoleukodystrophy with Combinatorial AAV-based Gene Therapy:
A Promising Approach for Restoring Myelin Function.
Adrenoleukodystrophy (ALD/AMN) is a devastating genetic disorder characterized by the progressive destruction of myelin, the protective sheath surrounding nerve fibers. This degeneration leads to neurological impairments, ranging from behavioral problems to muscle weakness and ultimately, death. While current treatment options provide limited relief of symptoms, they fail to address the underlying cause of the disease.
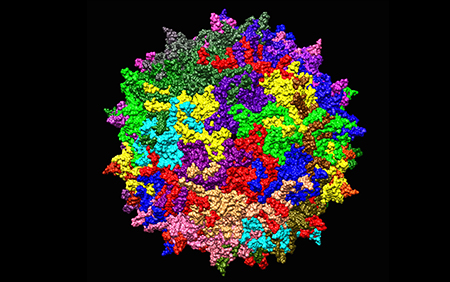
A promising new approach to treating ALD/AMN involves the use of combinatorial AAV-based gene therapy. This strategy combines the delivery of multiple therapeutic genes using adeno-associated viruses (AAV), a family of non-pathogenic viruses that can efficiently deliver genes to specific cells.
One of the key therapeutic genes targeted by this approach is the ABCD1 gene, which encodes an ATP-binding cassette transporter protein responsible for transporting fatty acids across the mitochondrial membrane. Mutations in the ABCD1 gene lead to the accumulation of harmful fatty acids in the brain, contributing to myelin damage.
AAV vectors can be engineered to carry and deliver functional copies of the ABCD1 gene to affected cells, potentially restoring the normal function of myelin-producing cells (oligodendrocytes) and halting the progression of ALD/AMN.
In addition to gene therapy, vectorized antibodies, also known as nanobodies, are being explored as a potential therapeutic strategy for ALD/AMN. Nanobodies are small, highly specific fragments of antibodies derived from camelids. They can be engineered to bind to specific proteins involved in myelin damage and prevent them from exerting their harmful effects.